"Questions Passed as Orders for Returns"-- When MPs ask, eventually someone is obligated to answer...
But you sure need a lot of patience waiting for the answers & they are so outdated! NOTE TO JOURNALISTS - YOU can take the questions below & start DIGGING NOW!
WARNING - this is a very long post that covers a lot of crucial territory.
;-)
When it comes to following our elected representatives in Ottawa, we can start with one of these two tools:
The Publication Search feature of the Government of Canada website found here: https://www.ourcommons.ca/PublicationSearch. Go to Hansard and type in the name of the MP you are following.
The OPENPARLIAMENT website where you can track speeches and comments made by the MP in committees or in the House of Commons. This is a volunteer, effort, run totally independently of government, great as a starting point because of its search features. Search by your favourite MP, or by key words, or write in “QUESTIONS PASSED AS ORDERS FOR RETURNS”. Then you can see what MPs are asking as they seek information on the different files they deal with.
By clicking on the blue Question line that shows up on OpenParliament, you can see the full set of questions being asked, plus the indicator number. That number will be important to try to track results later on.
To see if the questions have been answered (3 months after they were asked) it is likely best to ask the Information Service of the Parliament Library for help. https://lop.parl.ca/sites/PublicWebsite/default/en_CA/ContactUs
NOTE:
I have become aware of five Canadian Members of Parliament who have been using the mechanism of “Questions Passed as Orders for Returns” and “Questions on the Order Paper” to obtain important information from government offices that in my view should have been publicly available months and years ago. These five MPs (Colin Carrie, Dean Allison, Cathay Wagantall, Ted Falk, Leslyn Lewis) and their questions are copied in below. Some of the MPs have been receiving responses, but in the hustle and bustle of House of Commons politicking, it is not clear to me how the complete gathering of MPs ever gets to hear the responses to the government supplied answers when these are less than informative. So, with my background as a health advocate, public educator and resource developer, I provide my own questions/rebuttals to the weak answers presented as a result of the Order Paper questions. Feel free to share this post with anyone who seems unaware of holes in Government COVID-19 policies, despite its length, including our federal decision makers in the House of Commons and the Senate.
For further background & context on the whole COVID-19 debacle, also consider the four part “picture book” style explanation presented here:
The Order Paper Questions shown below SHOULD ALL HAVE been asked by our investigative journalists from the get go. As we tiptoe (or hurtle) towards new pandemics, health emergencies, global shocks or whatever else the globalists keep hinting about, it is important that we all, citizens, journalists, citizen-journalists, and the like, step up and do the digging… because by sitting around silently like we did four years ago, we will just get more of what we got before… a topsy turvy, poorly managed, outright dangerous and toxic health response.
Some of our MPs have figured it out. Encourage the others, as well as journalists and decision makers in your lives to start demanding answers before the practices and directives we experienced as the first COVID-response get locked into legislation on a permanent basis. Bill C-293 calls for more and more top down, anti-science, control-grid measures and even less “sober second thought”. EVERYONE OF US NEEDS TO PUSH TO GET ANSWERS FOR THE QUESTIONS BELOW. AND WHERE THE ANSWERS ARE UNSATISFACTORILY VAGUE, WE NEED TO KEEP PUSHING FOR BETTER ANSWERS, possibly even for admissions of wrongdoing the first time around. DO NOT ACCEPT FLUFF, CRAP OR BAFFLEGAB!
Back in June, MP Colin Carrie’s earlier questions were addressed (sort of). What he received shines a light onto the sorry state of science literacy at Health Canada. Whoever wrote the replies provided by the Parliamentary Secretary to the Minister of Health, Liberal MP Mr. Yasir Naqvi, is definitely NOT on top of the latest research presented by the Canadian and international trailblazers. Also the writer(s) is/are apparently NOT EVEN AWARE of the paperwork floating around within Health Canada/PHACs many offices, branches or divisions.
Here is just a taste of the information that the Ministry of Health could access if they only had the willingness to call in bona fide professionals with a focus on evidence-based COVID-19 science working in fields as diverse as computational biology, biochemistry, oncology, virology, vaccinology, and all manner of clinical practice.
Contamination of the Pfizer and Moderna COVID-19 modRNA vaccines by with high level of plasmid DNA and the presence of the SV40 promoter and enhancer, work done by Dr. David Speicher, Kevin McKernan and others (just ONE LINK of many)
Health Canada Hid Their Concerns About Impurities In COVID-19 Shots From Canadians (LINK)
British Columbia Centre for Disease Control Discovered COVID-19 Shots Were More Than Sixteen Times More Dangerous Than Flu Shots But Never Disclosed Publicly (LINK)
The elephant in the room that leads to a MORE THAN EXPONENTIAL INCREASE IN FEDERAL FUNDING FOR VACCINE INJURIES (LINK)
The FDA’s Backing Down on the Stance Against Ivermectin as an Effective Antiviral Agent (LINK)
Ontario mother Kayla Pollick experiences neck-down paralysis following a third dose of a modified/synthetic RNA shot. (LINK)
A group of leading medical researchers has uncovered evidence linking Covid mRNA shots to a recent surge in cases of lymphoma. (LINK)
Japan Warns COVID Vaccines Causing Global Population Collapse (LINK)
MEBENDAZOLE and LEUKEMIA - potent inhibitor of T-cell ALL Leukemia cells and overcomes chemoresistance. Mebendazole outperforms chemo (LINK)
Complete demographic switch and staggering surge in cancer cases among young people — “every new patient” now under 45 years old (LINK)
A situation report providing context for the World Health Organization’s declaration of global public health emergency for the Monkeypox (a sexually transmitted disease affecting very small localized populations) (LINK)
The fundamental importance of Vitamin D is demonstrated by the location of specific receptors for this essential vitamin in virtually every type of human cell, where they regulate hundreds of cellular processes to maintain good health. (LINK)
An analysis of Ontario’s own public health data showing that the vaccine mandates have completely failed to control COVID-19 case growth and that the fully vaccinated are contracting COVID-19 at higher rates than the unvaccinated. (LINK)
The importance of the refusal of the College of Physicians and Surgeons of British Columbia’s disciplinary panel to take judicial notice of certain facts in the disciplinary case against Dr. Charles Hoffe. (LINK)
Video footage showing contrasting claims re: pregnant and breastfeeding mothers made by leading Canadian public health officials which directly contradicted the data and evidence available from Health Canada and the vaccine manufacturers at the time (LINK)
Topics addressed at a single expert panel convened by the World Council for Health on a single day in June 2024 (LINK)
An update on the latest VAERS data, exploring how DNA contamination may lead to cancer on three different levels, and addressing issues related to genetic modification.
A closer look at DNA contamination - The debate is no longer about whether the 'vaccines' are contaminated; it's now about how significant the contamination is. (Presented by Human Genome Project researcher Kevin McKernan)
Revealing hidden promoters and enhancers in mRNA template plasmids, and exposing the spike protein as an estrogen receptor agonist with potential implications for breast cancer.
Potential integration and genotoxicity of vaxgenes in sperm cells - Exploring a new cohort survey on male fertility health and refining our scientific terminology related to mRNA gene therapy injections.
The house of cards is ready to fall - a litany of flawed science pulled together by retired microbiologist Dr. Sucharit Bhakdi (whom CBC, Wikipedia and others deemed as a provider of misinformation in 2020 when he and other international experts of his calibre started speaking out. EVERY SINGLE ONE OF THEIR CONCERNS has proven to be correct. Where are the apologies and retractions/corrections of false statements as per journalist ethics guidelines?)
The World Health Organization's disastrous handling of COVID-19, consistently advocating for misguided pandemic responses, has now evolved into a troubling bid for dominion over global health. It’s time to push back firmly with a resounding no.
Addressing the challenge of reaching the masses with accurate information amidst widespread propaganda and the demands of the attention economy.
Given the toxicity of LNPs - particularly with multiple doses over months or years - there should be an immediate global moratorium on the use of LNP-based so-called 'vaccines.'
The US HHS Power Grab: Exposing their plan to snatch unilateral power to declare public health emergencies, remove informed consent, quarantine US citizens and enrich the medical industrial complex.
Situation report on progress toward achieving justice: An in-depth update from an Australian attorney on legal proceedings in Australia and New Zealand, and their global implications.
Countless international experts are sharing the expertise at events continually being convened around the world to provide insight into the state of COVID/LongCOVID related research and their presentations are only a mouse-click away. And in Canada it takes OVER THREE MONTHS for MPs to simply get their questions answered — with pre-written statements that reveal incorrect and outdated understandings at Health Canada which is often 2-3 years behind the current evidence base. The answers received reveal hideously slow mechanisms at work. Below we copied out the questions being raised by MPs who seem AWARE of up to date research and who are trying to highlight the implications of this research for public health policy. But when those at Health Canada provide “answers”, they totally miss the mark, as if they are speaking from a different planet. This is the same planet that corporate backed “fact checkers” are on. Health Canada officials have consistently been invited to present the data they are drawing from to let it stand up against the rapidly evolving developments in the field AND to learn how to interpret the rapidly evolving data, but they have not consistently refused to do so.
When lives are at stake, it is NOT AN OPTION for bureaucratic slowness, regulatory capture and personal inability to admit having made deadly mistakes to mislead, misinform and misdiagnose the public and to continue doing so.
We should support those with the insight and courage to ask the important, difficult questions and we need to push back and demand better answers when those asking are eventually fed with spurious details or sweeping talking points - that all ‘MISS THE POINT’ and do not point toward a willingness to revamp health policy in line with ever-evolving scientific developments! Our government is pushing the envelope with futuristic research over at the Policy Horizons department. How can they be so deathly far behind in the Health Department?
[NOTE by FOLLOWING THE COVID SCIENCE: The question of the disconnect between knowledge being built at the frontlines of evidence-based science and the knowledge still being naively regurgitated in the cushy corner offices of government ministries was already driving my investigation prior to October 2021. At that time, I pulled together a COMPILATION OF LETTERS, ARTICLES & RESEARCH DOCUMENTS for the purpose of providing a record of the attempts by the “sidelined voices” to question the scientific basis of current policies and to providing guidance to action or judgement.
One piece of that compilation was essentially my “think aloud protocol” as I navigated through what the federal government funded CanCOVID project had deemed “Quick Links to Trusted Resources.” I was awestruck that someone was using our tax money to lead government health policy personnel AWAY from the widely regarded repository of published medical and scientific research papers (PUBMED) and to other sources deemed “trusted” for some reason. At that time Alberta was about to mandate COVID-19 injection for health care staff. YET on a repository from the WHO (which was included in the Trusted List) two studies appeared, one with an 85% efficacy rating of protection from COVID-19 for front line health care staff simply by taking 2 tablets of ivermectin. To follow my attempts to seek those same references in the other “trusted” sources (as per Health Canada designation), readers may view the first article in this collection. No wonder Health Canada officials were/are still (?) unaware of the body of evidence that supports the use of ivermectin as an effective infection prevention method. My hunt culminated in this insight:
When people limit themselves to choices made by others, when they limit themselves to these curated data sources, they should at least be knowledgeable of the selection criteria used - the questions that were being asked when the choices were made.
They must realize that they are allowing others to guide their knowledge-finding in a certain direction and away from another direction. But then they can no longer declare, as they have been, that others who follow the science directly, instead of via curated data sources that sift out various aspects of the field, are NOT following the science. It is they who are not following the science. (page 8)
Here now the many Questions on the Order Paper, with only a few having received official answers so far.
Question No. 2651— Mr. Colin Carrie — 2024-06-19
With regard to Health Canada (HC) and the initial Pfizer-BioNTech mRNA product and approval process thereof:
(a) did HC ask Pfizer to conduct genotoxicity studies to rule out insertional mutagenesis with DNA contamination;
(b) if the answer to (a) is negative, why not;
(c) what are the dangers with respect to insertional mutagenesis;
(d) in the context of the mRNA vaccine, what is the purpose of the lipid nanoparticle (LNP) delivery system;
(e) in the context of the mRNA vaccine manufacturing process, (i) what is the purpose of the SV40 enhancer-promoter-ori sequence, (ii) does it include a 72 base pair Nuclear Targeting Sequences (NTS), (iii) if the answer to (ii) is affirmative, what is the purpose of an NTS;
(f) with regard to the plasmid map used in the production of the modified mRNA, (i) on what date did the manufacturer provide the map to HC, (ii) what gene annotation was provided;
(g) in relation to (f), did the map contain an SV40 promoter-enhancer sequence and a reverse open reading frame;
(h) if no plasmid map was received, why did HC not ask for one; (i) according to the response to Order Paper question Q-2266, “There are strict limits and controls for the presence of these residual fragments to ensure that there is no effect on the safety or effectiveness of the vaccine,” as part of the residual DNA testing and measurement,
(i) what quantity of DNA fragments and SV40 enhancer-promoter fragments per dose were found in the Pfizer product, (ii) who provided the data to HC, (iii) when was this data provided to HC, (iv) is HC aware that the EMA reported a very large variance with respect to the residual DNA levels in the bulk mRNA and that the SV40 enhancer in the promotor sequence is 72 base pairs, (v) if the answers to (i) and (iv) are affirmative, what was HC’s appraisal of this information, (vi) what analytical techniques did the manufacturer rely upon to quantify the amount of RNA and the amount of DNA, (vii) do these quantities meet the “strict limits and controls for the presence of these residual fragments” and what are those limits;
(j) as part of HC’s requirements for lot release testing, has HC independently confirmed the quantity of residual DNA and SV40 sequences in the Pfizer-BioNTech product;
(k) if the answer to (j) is affirmative, (i) which laboratory and chief scientist provided this independent testing, (ii) what were the amounts recorded, (iii) were these different than those amounts provided by the manufacturer;
(l) if the answer to (j) is negative, why was independent testing not completed;
(m) is HC aware that Pfizer deliberately removed the SV40 enhancer sequence when reporting the annotated plasmid; and
(n) according to HC's response to Order Paper question Q-2266, “The SV40 promoter enhancer sequence… is inactive, has no functional role, and was measured to be consistently below the limit," (i) who provided HC with this assessment, (ii) is there evidence that the SV40 promoter binds to the P53 tumor suppressor gene and affects DNA repair mechanisms, (iii) if the answer to (ii) is affirmative, what are the risks to the health of Canadians as a result?
Response
RESPONSE & REBUTTAL by this lay person/public educator
[FOLLOWING THE COVID SCIENCE - In italics I will demonstrate to scientists, physicians, journalists and others how one cannot simply take what Health Canada officials tell us at face value when it is clear the processes they are following are not as rigorous as they and we would like to believe. After over 4 years of “following the COVID science” I can see where “official statements” are weak. What sounds like rants below are in all caps to help readers notice them. I am assuming the Parliamentary Secretary to the Minister of Health had help writing the responses he provides. It is inexcusable, however, that he and his department colleagues know less about ongoing developments in COVID-related scientific discoveries than interested LAY PEOPLE like me. Especially as Canadians continue dying and becoming disabled as a result of the COVID-related decisions made in the past and continuing to be made at this present time - We need to let our health officials see where they went wrong to avoid more wrongdoing!]
Mr. Yasir Naqvi (Parliamentary Secretary to the Minister of Health, Lib.):
Mr. Speaker, the health and safety of Canadians is Health Canada’s top priority, and the department exercises stringent regulatory oversight over vaccines.
Before the initial approval of the Pfizer-BioNTech vaccine, the department completed a rigorous scientific review of the product’s safety, efficacy and quality, including details on manufacturing processes and information on adverse events following immunization.
[FCSci - WHEN WAS THIS WRITTEN? WHERE IS HIS EVIDENCE OF A “RIGOUROUS” SCIENTIFIC REVIEW? THERE WASN’T ONE DONE IN CANADA. HE SHOULD BE ABLE TO PROVIDE THE ‘RIGOUROUS REVIEWS’, BUT THEY DON’T EXIST.]
An authorization was only issued once Health Canada confirmed the benefits of the vaccine outweighed the risks of its use.
[FCSci - DOES Mr. NAQVI HAVE ANY IDEA OF WHAT HAPPENED AT HEALTH CANADA re: lowering the bar for manufacturers? Does he know what they NO LONGER NEED TO SUBMIT, and how the regulators are now railroaded into acquiescing and giving authorization when they know they really shouldn’t/can’t? HAS HE EVER HEARD SHAWN BUCKLEY’s TESTIMONY at the NCI in Quebec City? https://nationalcitizensinquiry.ca/witness/shawn-buckley/ And how can a process that was launched as an EMERGENCY use order continue forming the basis of the drug approval process moving forward when there is no emergency? Why not at the very least return to the drug authorization process that was in place before 2019? (See reference below.)
The summary basis of decision can be found at this link: https://covid-vaccine.canada.ca/info/summary-basis-decision-detailTwo.html?linkID=SBD00510.
[FCSci - this leads to a blank template - with no where near enough categories of proof as would be necessary in a “RIGOROUS” procedure!]
During the review of the information submitted by the manufacturer, Health Canada became aware of the potential presence of residual DNA in the Pfizer-BioNTech mRNA vaccine preparation. However, the content of the residual DNA was below 10 nanograms/dose. The content of residual DNA was lower than the recommended limits established by the World Health Organization, WHO, in consultation with subject matter experts. Accordingly, the department did not request genotoxicity studies, as the residual DNA was considered a low risk.
[FCSci - RED FLAG WARNING - ANY JOURNALIST READING THIS SHOULD GET INFORMED ON THE DNA research (Speicher, McKernan, et al) and grill the speaker on this as this answer reflects a totally uninformed scriptwriter at work! It is high time that genotoxicity studies be a requirement at Health Canada given the recent revelations in this field of research.
Furthermore, the department reviews the manufacturing data for each vaccine lot that is released for use in Canada to ensure they meet their established specifications, including those for residual DNA.
[FCSci: WHAT???!! Am I reading this correctly here? Is this a pronoun substitution problem?The manufacturers have THEIR OWN specifications they must meet? OR does the government department which is supposed to authorize the products gave ITS own specifications? Or do the vaccine lots have their own specifications? Have you ever heard of food product labels and their “Recommended Daily Allowances” for various nutrients? Do the manufacturers each set their OWN standards there? Or is it a branch of government that sets them? WHY would it be different here?]
The amounts of residual DNA in Pfizer-BioNTech vaccine lots were reported to Health Canada, as well as to our international partner agencies: e.g., EMA and U.S. FDA. Both Health Canada and our international partners consider the test methods used by Pfizer-BioNTech for detecting that residual DNA to be scientifically sound and appropriate for their intended use.
[FCSci: OK, as per the government’s own “Trust and Transparency Strategy” since Health Canada considers “the test methods used by Pfizer-BioNTech” to be “sound and appropriate”, those methods should be posted publicly. What are they? Where can Canadians review them? Why would we let the INDUSTRY propose test methods for use with a product they plan to sell following their own test methods? And do other manufacturers also get to follow their own test methods that somehow Health Canada then also deems “sound and appropriate”? Wouldn’t the “rigorous scientific review” referenced by MP Naqvi entail more than checking if the manufacturer followed their own stated processes? Someone needs to hold those to account who pass this off as a solid response. ]
As well, the results from the residual DNA tests were consistent among different vaccine lots sold both in Canada and elsewhere and were also consistent with other manufacturing data provided in support of the release of each product lot.
[FCSci: Oh, so it’s OK if vaccines authorized in Canada have the same contamination as those being authorized elsewhere? By that metric, if other jurisdictions now see it fit to ban the use of the products in their jurisdictions, then Health Canada should also follow suit, right? THAT WOULD BE AN EXCELLENT IDEA. Is Health Canada studying the data that has led Dr. Ladapo in Florida to make the call to hold back mRNA injections? See the public statements here, and then be prepared to point at all the instances of misinformation perpetuated by the corporate backed ‘fact checkers’ who have the gall to make statements such as these found here:
Even with DNA vaccines, DNA integration is only a theoretical risk and has not been shown to be a safety problem.
“Moreover, we now have access to global surveillance data on over one billion doses of the mRNA vaccines that have been given, and there is nothing to indicate harm to the genome, such as increased rates of cancers,”
the available data on the mRNA COVID-19 vaccines show no sign of genomic disruption.
No corrections have yet been issued and obviously no attempts have been made to take down this uniformed content.
Returning to the response from Mr. Yasir Naqvi…
With regard to the Pfizer-BioNTech COVID-19 mRNA vaccine manufacturing process, an SV40 enhancer-promoter region was present in the DNA plasmid template, but has no functional role and is not upstream of any SV40 gene, and the DNA template is digested and filtered to remove from the mRNA vaccine. Health Canada cannot speculate on any intentions by Pfizer-BioNTech in regard to whether they removed the SV40 sequence when reporting the annotated plasmid. Please note that information requested on gene sequences, gene annotation and gene/plasmid map, received on November 16, 2020, as well as analytical techniques used by the manufacturer, are proprietary to Pfizer-BioNTech. However, Health Canada’s scientists reviewed this, and all of the other submitted information, and considered it within the risk assessment for their product. Any residual DNA fragments are considered as inactive, and an internal assessment of submissions by Pfizer-BioNTech found that they were consistently reported below the limit recommended by WHO.
Lastly, the lipid nanoparticle, LNP, component of the Pfizer-BioNTech mRNA vaccine preparation served as a delivery vehicle for the mRNA. Cells cannot efficiently internalize the mRNA in the absence of the LNP.
[FCSci - OK, so given the millions of copies of mRNA being injected which are propelled through the body inside their millions of LNP delivery vehicles, what does Health Canada know about the speed at which they travel… i.e. biodistribution data provided by Pfizer early on in its development process when it injected LNP ‘vehicles’ with a different ‘passenger’ into rodents? How soon did those ‘vehicles’ arrive in key organs of the body? And given that the experiment ended within a brief window of time, what would have happened to those rodents within days, weeks, months of these LNPs entering those organs had the animals been kept alive and the trial not ended so soon? Given that the LNPs themselves are made of something which, in turn, needs to be degraded into removable components that the lymphatic or endocrine system can eventually ‘clean up’, what is the cumulative effect, for example on the kidneys, of massive amounts of these components (like PEG) arriving en masse? And what about the so-deemed NOBEL PRIZE WINNING enhancement, to allow LNPs to be longer lasting than ‘normal’? Just exactly how long lasting are they? By which standard have Pfizer/Moderna et al demonstrated the pure harmlessness of degraded LNPs in the body? How long were any rats kept alive once they were subjected simply to LNP injections minus the mRNA content? And then what of other test animals whose body mass might be closer to humans and in which the larger concentrations of LNPs with and without mRNA content could have been tested? Are there any examples of sheep or pigs who have gone on to live healthy lives for 2+ years post injection? Or is something also causing those animals to drop dead in stunning numbers? Has it come to anyone’s attention that the massive increase in cancers and neurological conditions is showing up now months, and YEARS post injection? This is a far cry from the 28 day self reporting period that Health Canada seems to deem appropriate if that is part of the manufacturer’s “systematic” analysis of its own products!!! Is anyone at Health Canada aware of and following long range research? Or do the folks at Health Canada categorically deem such research as “misinformation” because it points to serious adverse events long after the manufacturer’s 28 day window? The long term sustained effect of single and multiple doses of mRNA + LNP must be examined BEFORE any more injections of this combination can possibly be “approved.” The currently recently announced APPROVAL must be RESCINDED until all of the issues outlined here have been more RIGOROUSLY examined!!
Question No. 2652—Mr. Colin Carrie:
With regard to Health Canada’s standards for safety and efficacy for the COVID-19 vaccines:
(a) have any COVID-19 vaccines met the requirements of Section C.08.001(2) of the Food and Drug Regulations (2)(g) and (2)(h) for safety and efficacy;
(b) has any COVID-19 designated drug or vaccine, approved under Section C.08.001(2.1) of the Food and Drug Regulations, subsequently met the standard for safety and efficacy as delineated in subsection (2)(g) and (2)(h) of Section C.08.001(2);
(c) if the answer to (b) is negative, why not;
(d) if a COVID-19 designated vaccine has not met (2)(g) and (2)(h) of C.08.001(2), which requires the sponsor to establish safety and efficacy, can the use of the terms “safe and effective” be applied to these vaccines;
(e) if the answer to (d) is affirmative, what is the rationale;
(f) with regard to the portal on the approval of COVID-19 vaccines for Comirnaty and available information for COMIRNATY - Submission control number 252736 on the Government of Canada's website, is the information for 2.7.1 Summary of Biopharmaceutic Studies and Associated Analytical Methods available to the public under the transparency initiatives;
(g) if the answer to (f) is negative, why not;
(h) as the mRNA vaccines represent a new manufacturing platform, do they meet the requirements of Section C.04.015 of the Food and Drug Regulations;
(i) if the answer to (h) is negative, why not;
(j) have the Pfizer-BioNTech and Moderna vaccines been assigned to Group 2 Lot Evaluation Group as part of the Lot Release Program; and
(k) if the answer to (j) is negative, why not?
Response
Mr. Yasir Naqvi (Parliamentary Secretary to the Minister of Health, Lib.):
Mr. Speaker, the health and safety of Canadians are Health Canada’s top priorities, and the department exercises stringent regulatory oversight over vaccines. Before any of the COVID-19 vaccines were approved in Canada, the department conducted rigorous scientific review of the extensive data regarding the vaccines’ safety, efficacy and quality, including results of pre-clinical and clinical studies, details on manufacturing processes, and information on adverse events following immunization. An authorization was only issued when the benefits of any of the COVID-19 vaccines outweighed the risks of their use, thereby allowing the use of “safe and effective” in describing the authorized vaccine preparations.
[FCSci - these examples of disinformation can’t be allowed to stand unchallenged. Can you make the risk/benefit analyses public? Do they differ by age, health status, body mass, pre-existing health conditions? Those analyses should form part of the public record along with all of the products listed by Health Canada. Easily clickable right on the page that lists each product.
Is the author of the response (and the person delivering it) even aware that there were TWO manufacturing processes involved? The one used for wide-scale product manufacturing was different from the small batch production initially used for the purpose of prototyping, testing and initial use. Is the speaker aware of the role of e-coli in growing commercial batches of product? I advise anyone at Health Canada making public statements about the vaccine manufacturing process to learn more to ensure what they say is grounded in fact, not conjecture:
https://nationalcitizensinquiry.ca/witness/dr-laura-braden/; https://nationalcitizensinquiry.ca/witness/deanna-mcleod/; https://nationalcitizensinquiry.ca/witness/deanna-mcleod-2nd-testimony/; https://nationalcitizensinquiry.ca/witness/deanna-mcleod-3rd-testimony/; https://nationalcitizensinquiry.ca/witness/kevin-mckernan/; https://nationalcitizensinquiry.ca/witness/dr-charles-hoffe/]
Mr. Naqvi is likely NOT AWARE of the success rate of the treatment protocol that Chinese, Italian, American, French, Mexican and other physicians were using to SUCCESSFULLY treat people and their families AT HOME, with quick recoveries for ALL OF THE MONTHS BETWEEN the arrival of SARS-CoV-2 and the announcement of the vaccines? How many months was that? What was happening to patients during that time? What were physcians doing? How many phyciscians downloaded the protocol put together by McCullough et al during those months? How many lives would have been lost if it wasn’t for that protocol? How many Canadian physicians were aware of and using the treatment approach described within that protocol WHO WERE THEN BANNED FROM CONTINUING TO TREAT PATIENTS when there was a need to demonstrate NO AVAILABLE ALTERNATIVES TO THE mRNA INJECTIONS in order to provide the major pharmaceutical corporations with so-called Emergency Use Authorization? How many ethical people at Health Canada etc. felt the need to resign instead of taking part in this sham? How many of the therapeutics in that protocol continue being used in countries that could not afford the costs of the mRNA injections and boosters? Is anyone at Health Canada even giving these questions any consideration? If not, why not?
Returning to the response to Mr. Carrie’s questions as provided by Mr. Naqvi:
The COVID-19 vaccines and treatments portal, https://covid-vaccine.canada.ca/, contains the summary basis of decision for each of the authorized COVID-19 vaccines.
[FCSci: a) Where are the afore-mentioned SBDs for each of the products on the list so that people can see on what basis the authorization was undertaken? b) There should also be links to the actual trial studies, the risk/benefit analyses, as well as the known contraindications that each of the products underwent if physicians and pharmacists are to provide their patients with the full information upon which to give fully informed consent. Contraindications should NOT simply be buried deep within the manufacturer’s product monograph but should be easily accessible from this page. Currently you only link people to the product monograph PRODUCED BY THE MANUFACTURER - not to a neutral third party or government approval document.
Notably, the product monograph states: “Patients should be advised of the nature of the authorization. For further information for NUVAXOVID® COVID-19 Vaccine (Recombinant protein, Adjuvanted) vaccine please refer to Health Canada’s COVID-19 vaccines and transfer portal. COVID-19 vaccines and treatments portal (canada.ca)” —> this causes health care providers to go round and round in circles since the Health Can page does not explain enough about the nature of the authorization.
In the product monograph I randomly opened, there are no links to the actual publication details of those studies, no names, no dates, no way for an interested pharmacist, for example, to compare and contrast products and results in a time effective manner. The document format throws around various trial studies in a helter-skelter manner. What info would Health Canada have been looking at to understand the mechanism of action of each of these products? Disorganized paperwork provided by the company seeking authorization can’t have been the ONLY thing Health Canada relied on, when making decisions around product approval, can it? Where is the rigour you are talking about?
Another question worth asking, is Health Canada not double checking on the studies that manufacturers are listing on their product monographs to ensure, for example that they all meet the requirement of at least 3000 trail and 3000 placebo participants (at the Phase 3 trial stage).
Clearly a number of data points listed in this monograph, particularly for the younger age range, do not appear to come from trials that meet that standard.
Is Health Canada aware of alternative trial designs that would provide higher quality results? Health Canada seems to be relying on clinical trials that, in turn, rely on “self reporting” of adverse events as the primary means of identifying adverse reactions. This while no one is briefing the public at large about the all-encompassing list of possible adverse events over every part of the body as identified early on by Pfizer. Who would suspect that, as in the case of this woman, a 52 year old former law enforcement officer, the puzzling onset of symptoms as varied as these could possibly all originate from one single cause?
Anemia, sleep apnea, overactive bladder, blood clots, brain fog, candida, dermatitis, hypersomnia, restless leg syndrome, tachycardia, oral lesions (erythematous) on the tongue, chronic tinnitus of both ears, pseudoparkinsonism, vertigo, not to mention Eppstein Barr virus, Myalgic Encephalomyelitis (Chronic Fatigue Symptom) AND MANY MORE? (LINK)
This one woman, when first reporting feeling unwell one month after receiving the “one and done” J&J shot was told by the physician that her symptoms related to menopause, low iron levels and insomnia. Two weeks after a subsequent booster, when experiencing hair loss and acne, the diagnosis was “stress”. Even if she had been a clinical trial participant, there would have been no ongoing monitoring or assessment of her (finally) correctly documented diagnosis as a vaccine-injured patient as some of the conditions took a number of years to completely manifest themselves and even longer to be diagnosed. All organs don’t just deteriorate on commando all at once. The toxic effects manifest themselves in different ways throughout the body. By the time monitoring would have stopped a few months in, this woman would have been considered a self reported case of “minor” adverse reactions - since the remainder of her valuable (yet horrible) patient history developed later on and was never documented or recorded anywhere in the official record that would eventually make it to people like MP Naqvi.
Given the lack of transparency around the wide array of adverse events following injection with a COVID vaccine on Health Canada’s website, Public Health Ontario took on the initiative to produce their own reference guide, a useful document that Health Canada would to well to post on its site too.
In contrast to a “self-reporting” trial design, is Health Canada aware of “before and after” trials? The teen boys in this study were not in a position to even sense, much less pinpoint the nature of the effects of the vaccine products on their cardiovascular systems. Were it not for the battery of tests taken before and some time after injection, the nearly one in three teens who had cardiovascular manifestations ranging from tachycardia or palpitation to myopericarditis would be proceeding in their lives at risk of a sudden onset of complications from slowly developing conditions. How many of the recent ‘sudden deaths’ in young men and women would have been avoidable has before and after (or at the very least after) screening be a routine follow up to these injections? The dubious “benefit” of the injection aside, at the very least being able to access regular follow up, testing and preventative thearpeutics following injection can save the lives of those who have become vulnerable as a result of the injections. Health Canada should stipulate before & after testing (of components like D-Dimer levels, troponin levels, etc.) in their requirements for trial design moving forward.
Also, why in 2024 is Health Canada authorizing a product that keeps referencing its trials on the Wuhan Strain? What about showing evidence that this product is to be effective with CURRENT STRAINS? And how is the word “effective” to be understood? Short term triggering of antibody production (the definition used in the first few months of the vax rollout) has proven to not be a reliable indicator at all. AND is it only Canadians not employed by Health Canada who took note of the statement by a high ranking Pfizer executive that their products were never tested on the criteria of preventing transmission? Do Health Canada employees still believe there is any good purpose for these injections? Can Health Canada document how they calculate the risk benefit analysis for the different population segments with a rigorous data review based exclusively on 2023/2034 data? Only then can we possibly understand Health Canada’s rationale for the elderly, the immunocompromised and Indigenous people to be currently injected? How is adding trillions of spike protein to the bodies of specifically these demographics supposed to be beneficial? How did these groups even end up on the target list for this product at this time?
And from a high-level perspective, what are we trying to accomplish? Most likely to have a healthy population? What is the evidence that these injections are the BEST way to attain that goal ? Where is the literature that Health Canada reviewed on other possible means of supporting population health? Are there recorded meeting minutes on decisions made at Health Canada NOT TO proceed with a comparative analysis comparing the use of vaccine products versus other means to see which approach leads to better health outcomes? And if such meeting notes do not exist, why not? What could possibly prevent Health Canada officials from considering ALL OPTIONS on the table prior to making the decision to move in one direction only when determining strategies for the best outcomes for the health of every Canadian?
Shockingly, the product monograph examined earlier has next to nothing re: contradictions and adverse events, for example here: “SERIOUS WARNINGS AND PRECAUTIONS At the time of approval, there are no known serious warnings or precautions associated with this product.” This is on page 5, but then on page 8: “Myocarditis and pericarditis have been reported following NUVAXOVID administration.”
AND ON PAGE 25:
DID ANYONE NOTICE THAT LAST STATEMENT? The MORE DOSES THE PERSON GETS THE MORE SEVERE THEIR SIDE EFFECTS??!!! That should be a RED FLAG to Health Canada and should prevent them from granting the authorization, as boosters typically get given repeatedly!!!!!
You can’t progressively change your statements as you progress further into the same document. What is true on page 25 must also be true on page 5.
Please note that biopharmaceutic studies and associated analytical methods are proprietary and cannot be disclosed.
[FCSci: WHAT???????????!!!!!!!!! If Health Canada is asking company X to ensure that Canadians will be SAFE, then Health Canada needs to demand company X test according to Health Canada’s standards. NOW YOU LET COMPANY X TELL YOU THAT THEY CAN’T TELL YOU HOW THEY TESTED THE PRODUCT? - No deal!!! If that is their attitude, you need to cancel the contracts. They work for you, you don’t work for them. (Unless we have already become fascist - where corporations tell governments how to set up their policies.)
All COVID-19 vaccines authorized by Health Canada under the interim order met the requirements of section C.08.001(2) of the food and drug regulations (2)(g) and (2)(h) for safety and efficacy. https://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._870/page-89.html#docCont
[FCSci - OK this is more like it. For EVERY ONE of the items listed on the COVID-19 vaccines and treatments portal, https://covid-vaccine.canada.ca, IN ADDITION TO the summary basis of decision for each of the authorized COVID-19 vaccines, there should be a link to the completed Food and Drug Regulation requirements package. Pharmacists and physicians should be able to look at every one of the required details. Unlike the haphazard product monograph, the Food and Drug Regulation requirements package creates a standard format against which each product can be compared with another on every one of the metrics. This Food and Drug document would need to be assembled based on submissions from the manufacturer where relevant but on the whole prepared by Health Canada staff.
No one buys a new car solely based on claims publised on the manufacturer’s website. There is a whole genre of neutral, third party consumer review literature that most people consult prior to making their choices. Health Canada neglects to provide this genre of information on these pharmaceutical products to provide the “consumer”, in this case the person consider taking the injection, or the pharmacist or nurse preparing to give the shot, with an unbiased source of information to consider as part of the informed consent process. WHY NOT?
Particular care must be given to include specific step by step descriptions of how the product works. This is the explanation provided by Health Canada:
We see here no indiction of HOW specifically cells are “provided with instructions”, of HOW specifically cells break down and get rid of the instructions and of HOW the cell “displays the protein piece on its surface”. No indication either of the processes before, during and after those steps along the way and NOTHING about the potential for interaction within the larger context of the body. Try this:
In the case of an mRNA injection…. the product contains approximately 200 million copies of mRNA instructions, each copy encased in its own lipid nanopoarticle “travel capsule”. Each capsule, once injected, is taken up by the tissues, and is moved around the body through the blood vessels and/or the lymphatic system. Where it stops moving, it ‘opens up’ and the mRNA instructions transfer by osmosis to the nearest cell (which can be an blood vessel lining /endothelial cell but can also be any cell type, for example a corneal cell, liver cell, brain cell or nerve cell). When the cell starts to manufacture spike protein, the immune cells such as macrophages are triggered to come attack the foreign spike protein. A caution is that they might also attack the body cell that makes the spike protein as per the mRNA instructions. The lipid nanoparticle capsule itself degrades. A caution is the ingredients of the capsule itself (It is made of … and …) as well as the fluid inside, which consists of PEG, x y and z.
Of course this example is simplistic, but not as simplistic as to lie by omission, which is what one could say about Health Canada’s oversimplistic explanation. IT HAS TO BE EASY ENOUGH FOR EVERY LAY PERSON TO UNDERSTAND AS PART OF THE UNINFORMED CONSENT PROCESS. BUT IT CANNOT BE MISLEADING. Still missing, when and how the antibodies develop, and when and where and how spike protein and the mRNA itself degrade. They are made to be long lasting, unlike the naturally occuring spike of the SARS-CoV-2 virus. What are the effects of having a few hundred thousand foreign spike protein float around the body. Where do they go? What do they do? Evidence now has spike protein lasting in the body for longer than 9 months. What can a physician tell a patient about the reason for putting so many spike protein in the body? Why weren’t just a few dozen or fewer sufficient? Is more truly merrier? With attenuated live virus, just a few bits were enough to produce immunity. Why do we NEEEEEED so MANNNNY spike protein? What is their purpose?
What is also missing is guidance for physicians as to how to provide all 4 requirements of informed consent. RISKS of taking the product, RISKS of NOT taking the product. BENEFITS of taking the product. BENEFITS of not taking the product…plus risks/benefits of doing x, y , z instead. This would include the risks & benefit of increased Vitamin D supplementation for those with Vitamin D deficiency. This could be a means of protection against all illness, not just COVID or the next Disease X - and also it would mean the ability to discuss all of the risks and benefits of the other products that you do NOT have on the chart of COVID therapeutics…
Currently we still have physicians fighting in court to uphold the right to confidential doctor patient conversations without the intrusion of the provincial colleges to declare which therapeutics are NOT TO BE DISCUSSED. Despite all evidence to the contrary, man still believe this is a free country and that healthcare cannot be highjacked at the whims of some private/public partnership somewhere else.
Currently posted on https://covid-vaccine.canada.ca/comirnaty-omicron-xbb15/product-details we find a product monograph from Pfizer posted on Sept 28 2023 which provides this NON-ANSWER to the question of how the product works:
Imagine paying over $20,000 for a new car based solely on this description provided by the manufacturer: “This vehicle uses gasoline to propel people forward. It also has a new design to ensure the safety of the driver and passengers while on the road. The vehicle is steered by a steering wheel and a drive train controlling four wheels. As with any vehicle, the design may not fully protect all those seated inside. Even after you have purchased the vehicle, continue to follow the recommendations of a dealer-certified mechanic to prevent accidental harm.”
Just as the car buying public would require MUCH more information, so also every medical professional who provided these injections to their patients required MUCH MORE INFORMATION. Where on Health Canada or PHAC’s public facing website was the basic mechanism of action outlined? How do Health Canada/PHAC officials explain the difference between mRNA/LNP technology and “conventional” attenuated live virus vaccines? There is NOTHING about that on the product details page. Why would interested parties seeking to make a determination about the advisability of certain vaccine products need to spend valuable time to go hunting elsewhere on Health Canada’s website in order to finally find https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/type-mrna.html. And why are live attenuated vaccines NOT EXPLAINED? Why did the writers at Health Canada instead compare mRNA vaccines with LIVE vaccines which apparently CAUSE infection!!
Short of being a technique used in psychological operations to cast the non-desired option in a negative light to purposefully nudge the reader towards the desired (more lucrative option), how else can the statement “mRNA vaccines are not live vaccines and cannot cause infection in the host” possibly be explained? There is NO SUCH THING as a “LIVE VACCINE” UNLESS the reference is to a LIVE ATTENUATED VACCINE. This is how our American counterparts went about explaining the difference between the vaccine type used BEFORE COVID-19 (live-attenuated) and the “new technology” of mRNA.
Even Pfizer doesn’t use the term LIVE vaccine in such a way to insinuate that such a vaccine causes infection in the host.
Why is it that when the question comes up I need to refer interested people to MY OWN EXPLANATION of mRNA technology because this CRUCIAL UNDERSTANDING is not included in an honest and straight-forward manner on our government health webpages? (Or is it now finally? I would love to stand corrected!!)
Did anyone notice that Health Canada did NOT answer a number of very specific questions? MP Carrie asked about:
C.04.015 On written request from the Minister, every fabricator, packager/labeller, tester, distributor referred to in paragraph C.01A.003(b) and importer of a drug shall submit protocols of tests together with samples of any lot of the drug before it is sold, and no person shall sell any lot of that drug if the protocol or sample fails to meet the requirements of these Regulations.
[FCSci: Where does Health Canada store the samples of lots of the drug/injection? The link to that protocol should be made part of the record that any pharmacist or physician can access when going to the Health Canada site. Where on Health Canada’s site are the protocols which manufacturers need to follow? Pre-Covid Health Canada required trials with a total of at least 3000 test subjects and 3000 control subjects. Following along with the “Agile Regulations” focus so beloved by the World’s largest corporate lobby group, it seems Health Canada has caved into doing what is best for BigCorp - look carefully and you will see a clear desire for red tape and “unnecessary bureaucratic activities” to be reduced to allow pharmaceutical companies to be less encumbered in their application processes - It is clear that the the expense and time delays involved in truly representative clinical trials (and the risk that the results don’t turn out as desired) led to a great desire on the part of BigCorp to lobby governments to cut down their requirements for longer term proof of safety studies. The new favourite buzzword in the boardrooms of the world’s largest corporate lobby group “agile” returns over 3500 hits. Of note is that lobby group’s “Toolkit” for Government Regulators entitled “Agile Regulation for the Fourth Industrial Revolution”. When more Canadians learn of attempts to formalize the REDUCTION OF SAFETY requirements because that is so much more convenient for Big Pharma, and the vast array of documentation produced by the World Economic Forum intended as Toolkits, Guides and Handbook of corporate-friendly policies on nearly every aspect of the economy to be implemented by compliant and accepting governments, they will provide pushback like you have not yet seen!]
Terms and conditions were imposed upon the authorization with respect to quality, clinical, labelling, and risk management plan requirements. This process has allowed Health Canada to assess information submitted by the manufacturer as it became available during the product life cycle to ensure that the benefits of the vaccines continued to outweigh the risks. Additional safety and effectiveness data generated post-approval continue to support Health Canada's original decision.
[FCSci: OK so what are you doing with all the NEGATIVE safety and efficacy data that has now been amassed WORLD WIDE post-approval? It certainly doesn’t support Health Canada’s original decision. What pressures are you under to pretend that all is well? What would happen if you were to pull the plug on these products moving forward? The population needs an easy fix. Here is one, something you can “save face” on: https://www.ourcommons.ca/petitions/en/Petition/Details?Petition=e-5076
1. Raise vitamin D blood level targets and out-dated guidelines;
2. Encourage provincial partners to remove disincentives for vitamin D blood tests; and
3. Promote vitamin D sufficiency for all Canadians.]
Lastly, the mRNA COVID-19 vaccines authorized by Health Canada met the requirements of section C.04.015 of the food and drug regulations. However, the lot evaluation group assigned to mRNA vaccines, as part of the lot release program and protocols of testing submitted by the manufacturers, is proprietary and cannot be disclosed.
[FCSci: While details on the “recipe” might be “proprietary”, when it comes to testing, the proof is in the pudding. The whole world needs to see that the different products can stand up to the clearly stated standards. The protocols of TESTING MUST BE OPEN FOR ALL TO SEE.
Consider this example: I raced this car to X kilometres, and slammed on the brakes at x speed and it took X seconds to stop. EVERY CANDIDATE MUST MEET THE SAME STANDARD and that needs to be open for all to see. Can you tell how keeping things secret protects the manufacturer and not the public? We are well on our way to having BigCorp shape government policy. After WWII there were posters everywhere: NEVER AGAIN FACISM! Health Canada has slipped down a slippery slope. You need to take your power back - no way can BigPharma be the tail that wags the government dog!]
MORE QUESTIONS RECEIVING REPLIES
https://www.ourcommons.ca/documentviewer/en/44-1/house/sitting-346/order-notice/page-9 shows all the outstanding questions carried over from earlier in the year. Questions that have already received answers apparently get dropped from the list.
MP Cathay Wagantall - Starting to get answers from Stats Can & PHAC
About three months after Yorktown SK MP Cathay Wagantall requested information, she received responses from Statistics Canada and the Public Health Agency of Canada.
parl-gc.primo.exlibrisgroup.com/discovery/delivery/01CALP_INST:01CALP/12165649990002616?lang=en
This is what she was asking (Q2741)
With regard to Statistics Canada’s (StatCan) released data regarding "provisional deaths and excess mortality in Canada" which reported "significant excess mortality starting in January 2022" especially “among individuals younger than 45” and the Privy Council Office’s (PCO) use of “Winning Communication Strategies” to “not shake public confidence” (ATIP, May 2021):
(a) why did StatCan wait until September 2022 to publish excess mortality data amongst young Canadians when the data was available around March or April 2022;
(b) who signed off on the data in (a);
(c) what steps were taken to investigate the underlying reasons for this unusual finding of excess deaths in young persons;
(d) who or what agency or entity informed the Office of the Prime Minister and the Cabinet about this finding;
(e) how and when were these statistics communicated to provincial and territorial health ministers, regulatory health care colleges, chief medical officers and
coroner's offices, in order to provide Canadians with updated data to facilitate informed consent;
(f) which officials at which agency or entity hosted press releases regarding this unusual rise in deaths among those Canadians under the age of 45 years;
(g) as per the Public Health Agency of Canada’s ‘Cases Following Vaccination’ reports from June 10, 2022 to September 23, 2022, what was the number of “COVID-19
Cases Deceased” for each week as of the week which ended on June 12, 2022 until the week which ended on August 28, 2022, broken down by the vaccine status of the individual, including those having received (i) no dose, (ii) a single vaccine dose, (iii) the primary program of two doses, (iv) one additional dose, (v) two additional doses;
(h) according to the numbers in (f), which group had the largest number of
“Cases deceased” each week;
(i) specifically with respect to the unvaccinated group and the two additional doses group, during those weeks, which of these two groups demonstrated fewer COVID-19 outbreaks;
(j) were there any press releases communicating the findings in (i) to the public;
(k) what are the details of the memo drafted by the PCO in May 2021, that instructed recipients to skew statistics to minimize the impact of vaccine-related deaths or injuries, including (i) which agencies or entities and which specific officials received this memo, (ii) how did the agencies or entities carry out the PCO’s instructions vis-a-vis statistical skewing, (iii) who at each agency or entity signed off on
the report of the data;
and (l) why is there a discrepancy between the data that was released on the StatCan website for “other ill-defined and unspecified causes of mortality” from 2020 to 2022, a reported 16,043 deaths, and the value provided in the government response to Order Paper Question Q-1115, of 55,975 deaths for the same year and same category?
[FCSci: I will leave it to others to critique these responses.]
REPLY
(from: https://parl gc.primo.exlibrisgroup.com/discovery/delivery/01CALP_INST:01CALP/12165649990002616 with highlighting added by FollowingTheCovidScience.)
Part a) Statistics Canada did not wait until September 2022 to publish excess mortality data among young Canadians. Excess mortality among those under the age of 45 was observed at different times throughout the pandemic, a fact that was acknowledged and explored in the April 2022 release as well as in many of Statistics Canada’s earlier monthly analyses of excess mortality
(https://www150.statcan.gc.ca/n1/daily-quotidien/220414/dq220414d-eng.htm).
For the March and April 2022 releases, insufficient data had been received from most provinces and territories to comment on excess mortality at a national level beyond November 2021. Among those jurisdictions for which data were available, excess mortality was observed in some provinces in January 2022.
The May 2022 release did recognize that excess mortality was increasingly impacting younger Canadians in early 2022 (https://www150.statcan.gc.ca/n1/daily-quotidien/220512/dq220512c-eng.htm), an observation that was repeated in the June, July, and August releases.
In its commitment to keep Canadians informed on the direct and indirect consequences of the COVID-19 pandemic, Statistics Canada began, in May of 2020, publishing monthly provisional data on excess mortality in Canada. These releases were announced in The Daily, Statistics Canada’s official release bulletin, and accompanied by an analysis by region, sex and/or age group to provide
some insight as to what groups were most impacted by the pandemic. The data are provisional as they are not based on all deaths that occurred during the reference period, owing to reporting delays. Canada's national vital statistics system is a complex and decentralized system, based on collaboration between provincial and territorial vital statistics registrars and Statistics Canada. The Vital Statistics Registry in each province and territory registers all deaths occurring in their jurisdiction and reports the information to Statistics Canada. Statistics Canada's capacity to provide useful and
timely information is dependent on when the information is reported by the provinces and territories.
The numbers of excess deaths discussed in the monthly releases refer to provisional estimates; these estimates are updated with revised figures as more data are reported by the provincial and territorial registries.
Part b) In accordance with Statistics Canada’s Policy on Official Release, the Chief Statistician is responsible for performing the final step of the institutional review for all content proposed for publication in any of Statistics Canada's official release vehicles (https://www.statcan.gc.ca/en/about/policy/official_release). Additional information on the roles and responsibilities with respect to the approval process are described in Section 5 of the Policy.
Part c) In the absence of timely data on the causes of death through the civil registration process, data sources external to Statistics Canada were considered to provide context to the increased mortality observed in those under the age of 45. The offices of the provincial and territorial chief coroners and chief medical examiners in some jurisdictions—notably Alberta and British Columbia—were publicly reporting an increase in the number of overdose deaths during the pandemic, particularly among younger Canadians. Furthermore, our own data on COVID-19 deaths, corroborated by those collected by the Public Health Agency of Canada, showed that very few deaths
among Canadians aged 0 to 44 years were attributed directly to the disease. The data collected and published by the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) were also consulted: according to its most recent data, which include adverse events having occurred on or before January 5, 2024, 4 deaths were consistent with causal association to immunization
(https://www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html).
Deaths investigated by coroners or medical examiners, such as homicides, suicides, and accidental death, including drug overdoses, often require lengthy investigations, which means it takes longer for these causes of death to be reported to Statistics Canada. While the monthly releases include provisional death counts by the cause of death, these data represent an underestimation of the total number of deaths and the number of deaths attributable to selected causes of death during the reporting period. When the cause of death information is delayed from the provinces and territories and/or by means of an investigation by a coroner or medical examiner, challenges arise in attributing decreases and increases in mortality to a particular cause or causes. Statistics Canada publishes annually more comprehensive data on the causes of death, usually in November or December of the following year, the most recent having
occurred on November 27, 2023, with the release of data on deaths and the causes of death for the 2022 reference year (https://www150.statcan.gc.ca/n1/daily-quotidien/231127/dq231127b-eng.htm).
These figures are also subject to annual revision as new and updated information on the causes of death is made available to Statistics Canada.
Part e) Statistics Canada does not communicate directly these statistics to provincial and territorial health ministers, regulatory health care colleges, chief medical officers and coroner's offices. The information is publicly available on the Statistics Canada web site (https://www.statcan.gc.ca/en/start).
Part l) The 55,975 deaths attributed to Ill-defined and unspecified causes of mortality (for reference year 2022) in the statistics provided in the response to Order Paper Question Q-1115 are based on the provisional data released on December 8, 2022 (https://www150.statcan.gc.ca/n1/daily-quotidien/221208/dq221208f-eng.htm); 16,043 is the number of deaths that occurred in 2022 and for which the underlying cause of death remained undetermined or unknown in the comprehensive data on the causes of death for 2022, released on November 27, 2023. The cause of death category Ill-defined and unspecified causes of mortality is used when the leading underlying cause of death is undetermined or unknown. Deaths are assigned to this category when: the investigation failed to conclusively establish a specific cause of death—i.e., the medical certificate of cause of death
It looks like question f) fell through the cracks.
Public Health Agency of Canada
Part (g), (h), (i) and (j):
(g) Between June 10 and August 28, 2022, the reporting of vaccination status categories were updated on the COVID-19 epidemiology update webpage to reflect updated terminology and relevant vaccination statuses at the time. As such, deaths by vaccination status categories are not directly comparable when definitions changed, resulting in gaps of data for the weeks of June 28 to July 4 and July 19-24. The following data was grouped according to vaccination status categories as presented on the COVID-19 epidemiology update webpage.
Based on “Cases following vaccination” reports on the COVID-19 epidemiology update web page from June 10 to August 28, 2022, weekly COVID-19 deaths were as follows:
• Between June 13-27, 2022, the average number of weekly deaths reported was 23.3 among unvaccinated cases, 1 among not yet protected cases, 8.3 among partially vaccinated cases, 73.3 among fully vaccinated cases, and 144 among fully vaccinated cases with an additional dose. (3 injections)
• Between July 4-18, 2022, the average number of weekly deaths reported was 13 among
unvaccinated cases, 23.5 among fully vaccinated cases, 63 among fully vaccinated cases with one additional dose, and 20 among fully vaccinated with two or more additional doses.
• Between July 25 – August 29, 2022, the average number of weekly deaths reported was 28.7 among unvaccinated cases, 7 among cases with primary series completed, 109.3 among cases with primary series completed and one additional dose, 46.3 among cases with primary series completed and two or more additional doses.
(h) Across all weeks in the time period of interest, the number of deaths were highest among those with a primary series and 1 additional dose.
For parts (g) and (h) please interpret the numbers provided with the following considerations and data caveats: Please note that these are crude numbers and do not account for the number of people in each vaccination status category. During the timeframe of interest, those with a primary series and 1 additional dose were the largest vaccine status group in the Canadian population (approximately 50% of the Canadian population and increasing). As such, although the crude number of deaths are highest among this group, the rate of deaths among those with a primary series and 1 additional dose are low in comparison to those who are unvaccinated. (Those unvaccinated were 8 times more likely to die than those with a completed
primary vaccine series and 1 or more additional doses. From the August 26, 2022 COVID-19 epidemiology update web page.)
Furthermore, case counts are likely to over-represent people at risk of severe disease, because they have been prioritized for testing. These same people were also prioritized for COVID-19 boosters. This meant that the people being tested were more likely than the general population to 1) have received boosters, and 2) to get severe illness. This leads to a data bias which could cause people to mistakenly conclude that
more vaccines lead to severe disease. As they are a larger group of people, there will naturally be more cases among vaccinated people than among unvaccinated people. However, despite their higher case counts, vaccinated people are less likely to get very sick or die than those unvaccinated for COVID-19.
Parts (i) and (j) PHAC does not collect data on outbreaks specific to vaccination status. As a result, this is not reported to the public, nor were there press releases.
FollowingCovidScience notes that the data coming out British Columbia, but later taken down (recovered and shared by US vaccine safety advocate Steve Kirsch) supports the findings shared re: Canada as a whole. Roughly 5.3 million people had one or more COVID-19 injections, 52% had 3 or more doses at the time. All things being equal, they should represent about 50% of the other categories as well. By general logic, those with 3 or more shots should be MORE protected from hospitalization, critical care status and death. Yet in all three cases, it is those with the most injections who top the chart.
MORE QUESTIONS AWAITING HONEST ANSWERS
MP Cathay Wagantall - re: COVID-19 Therapeutics Task Force (TTF)
On June 19, MP Wagantall is listed here as also having asked these questions:
Questions Passed as Orders for Returns With regard to the COVID-19 Therapeutics Task Force (TTF) who oversaw submissions for grant funding from Innovation, Science and Economic Development Canada (ISED)’s Strategic Innovation Fund:
(a) in total, how many projects were considered for funding;
(b) with respect to the projects which were funded, (i) how many received funding, (ii) how much funding was allocated per project, (iii) which drugs were being investigated per each approved project, (iv) what was the total amount of funding granted for the approved projects;
(c) with regard to the projects which were not approved for funding, what recommendations were made to them;
(d) with regard to the therapeutics which were recommended for purchase, (i) what were these therapeutics, (ii) were these therapeutics purchased, (iii) what was the implementation plan, (iv) if there was no plan, why not;
(e) were the drugs Ivermectin or Hydroxychloroquine considered by the TFF;
(f) if the answer to (e) is affirmative, what were their recommendations and how did they arrive at them;
(g) who were the members of the TTF;
(h) were any of the members pharmacists, pharmacologists, or toxicologists;
(i) what were the members' conflicts of interest;
(j) did any of the members withdraw from the task force prior to its conclusion;
(k) if the answer to (j) is affirmative, who left early and why;
(l) regarding the document entitled “HEALTH CANADA/ PUBLIC HEALTH AGENCY OF CANADA MEMORANDUM TO THE MINISTER OF HEALTH, Meeting with the COVID-19 Therapeutics Task Force” dated February 24, 2021, and signed by the President of the Public Health Agency of Canada and the Deputy Minister of Health, which reads that “At the previous meeting TTF members expressed concern that their mandate was ending.
MP Cathay Wagantall - re: Vaccine Injury Support Program
Questions on the Order Paper With respect to Canada’s Vaccine Injury Support Program (VISP):
(a) how many claims have been filed to the program from December 8, 2020, to present day, broken down by age group;
(b) how many of those claims have been approved, broken down by age group;
(c) of the approved claims, what have been the diagnoses and their frequencies, broken down by age group, date approved, and the corresponding COVID-19 vaccines that were administered;
(d) of the approved claims, what are the percentages of Canadians who received (i) the AstraZeneca COVID-19 vaccine, (ii) the J&J; COVID-19 vaccine, (iii) any COVID-19 vaccine produced by Pfizer-BioNTech, (iv) any COVID-19 vaccine produced by Moderna, (v) a combination of COVID-19 vaccines;
(e) how many persons have received compensation to date through the VISP;
(f) what is the total compensation to date given to vaccine-injured Canadians;
(g) what is the age of the youngest person who received funding support approval through the VISP, and their associated diagnosis;
(h) for all death claims, (i) what is the total number of death claims that have been filed to VISP, (ii) of the total, what have been the underlying causes of death, aside from the vaccine and their frequencies, (iii) how many filed death claims have been approved by the VISP and their corresponding diagnosis and vaccine status;
(i) did the VISP require autopsies prior to approving a death claim;
(j) if the answer to (i) is affirmative, what immunohistochemistry requirements does the VISP specify for these autopsies;
(k) when denied, how many persons have appealed their claim and how many have been successful;
(l) regarding the determination of causality of the adverse event in relation to a COVID-19 vaccine, (i) what is the standard criteria, (ii) does the Medical Review Board take into consideration the Bradford Hill criteria;
(m) what are the professional qualifications of each member on the Medical Review Board; and
(n) who are the professionals on the Medical Review Board?
MP Cathay Wagantall - re: possible communications with ON College and/or Health Professions Regulatory Advisory Council
Questions on the Order Paper With regard to Health Canada (HC), the Public Health Agency of Canada (PHAC), the National Advisory Committee on Immunization (NACI) and any communications in 2020 or 2021 regarding mask exemptions, COVID-19 vaccines, medications to treat COVID-19, or any other public health messaging about COVID-19:
(a) did (i) the Minister of Health, (ii) the Deputy Minister of Health, Dr. Steven Lucas, (iii) the Chief Public Health Officer, (iv) the Deputy Chief Public Health Officer, (v) the Chief Medical Officer at Health Canada, (vi) any personnel from HC, (vii) any personnel from the PHAC, (viii) any personnel from the NACI, (ix) any firm contracted by or through HC, PHAC, or NACI, communicate or correspond, directly or indirectly, with the College of Physicians and Surgeons of Ontario (CPSO);
(b) if any of the answers to (a)(i) through (a)(ix) are affirmative, (i) when did these communications occur, (ii) what are the summaries of those communications;
(c) did any of the individuals or agencies in (a)(i) through (a)(ix) of (a) communicate with the Health Professions Regulatory Advisory Council (HPRAC); and
(d) if the answer to (c) is affirmative, what are the summaries of those communications?
MP Cathay Wagantall - re: mixing and matching of COVID-19 vaccines
Question 2852 — September 16, 2024 — Ms. Cathay Wagantall
With regard to government approval of the mixing and matching of COVID-19 vaccines (heterologous vaccination):
(a) what data did the manufacturers of the Pfizer, Moderna and AstraZeneca COVID-19 vaccines have with respect to mixing their products with other COVID-19 vaccine products;
(b) in mid-2021, when Health Canada (HC), the Public Health Agency of Canada (PHAC) and the National Advisory Committee on Immunization (NACI) were recommending mixing vaccines to Canadians, what did the Pfizer, Moderna and AstraZeneca COVID-19 vaccine monographs recommend at that same time;
(c) what scientific rationale did HC, the PHAC and the NACI have for heterologous vaccination, broken down by (i) Pfizer mRNA vaccine and Moderna mRNA vaccine, (ii) mRNA vaccine and adenovirus vaccine;
(d) what advice or instruction did the government receive from the World Health Organization’s Chief Scientist, Dr. Soumy Swaminathan, regarding the safety and efficacy of this approach in July 2021;
(e) with respect to the advice in (d), did HC follow that advice and, if not, why not;
(f) what data regarding the safety and risks of heterologous vaccination in Canadians (i) did the PHAC, the NACI or HC have at the time mixing was recommended, (ii) does the PHAC, the NACI or HC have currently;
(g) with respect to the Canadian study related to the mixing and matching of COVID-19 vaccines, when will the results of the MOSAIC trials (CT24) NCT04894435 sponsored by the Canadian Immunization Research Network become available;
(h) with respect to the study in (g), what are the interim results; and (i) with respect to the study in (g), what are the final results, if anything?
Other MPs are listed here as having asked these questions:
MP Dean Allison re: Health Can “approval” of C-19 vaccines and subsequent vaccine and travel mandates
[Following the Covid Science asks: How is it that the questions here are only being asked NOW given that vaccine mandates in the federal public sector date back to October 2021 - why was NO ONE asking about the “the immunological mechanism of action of the COVID-19 mRNA vaccines that enables them to stop the spread of SARS-CoV2” at the time? Or even better, why was Health Canada not expelling this to the public PRIOR to mandating that federal public sector workers take them? Did Health Canada not think that ITS OWN STAFF should understand what they were letting be injected into their own bodies?]
Q-29162 — June 18, 2024 — Mr. Allison (Niagara West) — With regard to Health Canada's (HC) decision to approve the COVID-19 modRNA vaccines and the Prime Minister's subsequent support for the vaccine mandates in the federal public sector and vaccine passports for travel purposes during the COVID-19 pandemic:
(a) what is the immunological mechanism of action of the COVID-19 mRNA vaccines that enables them to stop the spread of SARS-CoV2;
(b) what data supports this mechanism of action;
(c) who or what agency provided the data and verified the data;
(d) when was this data provided to (i) HC, (ii) the Office of the Prime Minister;
(e) what data did Pfizer and Moderna produce to HC that demonstrated (i) the period of time the spike protein is produced in the body, (ii) where in the body the spike protein is produced; and
(f) in relation to (e), what was the period of time Pfizer and Moderna tracked the spike protein in their clinical studies?
MP Ted Falk re: Vax Approval for 12-15 year olds
Q-29172 — June 18, 2024 — Mr. Falk (Provencher) — With regard to Health Canada's (HC) establishing the safety of the Pfizer/BioNTech COVID-19 vaccine in 12-15 year olds:
(a) what serious adverse events (SAEs) did the pharmaceutical company disclose to Canada's health agencies for this age group pre-authorization;
(b) since approving the product in this age group, has the Public Health Agency of Canada (PHAC), the National Advisory Committee on lmmunization (NACI) or HC become aware of additional adverse events (AEs) or SAEs that the pharmaceutical company had not disclosed during the initial authorization process;
(c) if the answer to (b) is affirmative, (i) what AEs and SAEs has the PHAC, the NACI and HC become aware of, (ii) when were they discovered, (iii) what are the means by which Canada's health agencies were provided this information;
(d) prior to authorizing this product in this age group, was the PHAC, HC or the NACI given information about (i) the SAEs of a 12-year-old trial participant named Maddie de Geray who was diagnosed with chronic inflammatory demyelinating polyneuropathy which rendered her reliant on a wheelchair and feeding tube, (ii) any other specific SAE cases in this cohort following the Pfizer inoculation;
(e) if the answer to (d) is affirmative, what was the date and means by which the PHAC, the NACI or HC became aware of these cases;
(f) if the answer to (d) is negative, has Ms. De Geray's diagnoses been added to HC's list of SAEs on the HC website in this age group;
(g) has the PHAC, HC or the NACI been aware that the adverse events experienced by trial participant Maddie de Geray were not properly disclosed within their trial studies as described in the scientific publication of said trial (i.e. NEJM - Frenck et al. 2021);
(h) did the PHAC, HC or the NACI take any action after discovering the lack of proper disclosure of Maddie de Geray's SAEs by Pfizer;
(i) what was the age stratified risk-benefit analysis for 12-15 year olds in relation to the Pfizer/BioNTech COVID-19 vaccine at the time of authorization, on May 5, 2021;
(j) what was the data and calculations for quantifying the risks and benefits that Canadian health agencies used to authorize or approve the product in this age group;
(k) what data indicated that the benefits of the vaccine outweighed the risks at the time of authorization;
(l) since the roll-out of the Pfizer/BioNTech COVID-19 vaccine in this age group, (i) what are the top ten SAEs identified in this cohort, (ii) how have these SAEs been communicated to the medical community and the public at large;
(m) what type and frequency of SAEs in 12-15-year-old would invoke an unfavourable benefits-risk ratio for healthy children and for children with underlying medical conditions; and
(n) is HC, the PHAC or the NACI aware of any other jurisdictions worldwide that no longer recommends the mRNA COVID-19 vaccines in children at (i) six months of age, (ii) between six months and two years (iii) between two to five years, (iv) between five to 12 years, (v) between 12-15 year, (vi) between 15-18 years?
MP Ted Falk re: specific nature of the nanotechnology of the lipid particles, pegylated LNPs, residual DNA and more
Q-29182 — June 18, 2024 — Mr. Falk (Provencher) — With regard to Health Canada's (HC) review of the COVID-19 modRNA vaccine products:
(a) did HC consider the specific nature of the nanotechnology of the lipid particles used for the modRNA vaccine delivery;
(b) if the answer to (a) is affirmative, what was their assessment;
(c) why was the fact that modRNA vaccines contain nanotechnology omitted from the product monograph-label;
(d) did HC assess the toxicity of pegylated nanoparticles, specifically the risk for complement activated related pseudo allergy (CARPA) with the lipid nanoparticles used in the mRNA vaccines;
(e) if the answer to (d) is affirmative, why was this not included in the product labelling;
(f) if the answer to (d) is negative, why wasn't this assessed;
(g) did HC assess the risk of toxicity due to the nanoformat of these vaccines;
(h) if the answer to (g) is affirmative, what was the assessment result;
(i) if the answer to (g) is negative, why not;
(j) did HC assess the lipid nanoparticles as a novel excipient;
(k) if the answer to (j) is affirmative, what was the assessment;
(l) if the answer to (j) is negative, why not;
(m) with respect to nanotechnology products and their unique properties and behaviors particularly in their application to the modRNA vaccines, did HC examine (i) the safety, (ii) the effectiveness, (iii) the risk to the environment, (iv) its specific regulatory status;
(n) if the answers to (m)(i) to (m)(iv) is affirmative, what were the assessment results;
(o) if the answers to (m)(i) through (m)(iv) is negative, why not;
(p) how do established safe levels of DNA apply, (i) when using pegylated LNPs as a delivery system, (ii) when a product that contains pegylated LNPs requires repeated dosing; and
(q) what assessment was performed to assess the risk of residual DNA when using pegylated LNPs as a delivery system in a vaccine which requires repeated dosing?
MP Leslyn Lewis re: ONE HEALTH approach + also MAID
Q-29782 — September 17, 2024 — Ms. Lewis (Haldimand—Norfolk) — With regard to the government’s response to the COVID-19 pandemic and the One Health approach: (a) has the government undertaken a formal and public review of Canada’s whole-of-government pandemic response to learn from the past and inform future national pandemic planning;
(b) if the answer to (a) is negative, what are the reasons;
(c) if the answer to (a) is negative, when will the government conduct a formal and public review of Canada’s whole-of-government pandemic response;
(d) how does the government define a pandemic;
(e) what is the government’s current policy with regard to the One Health approach;
(f) how does the government define One Health;
(g) if this term is not defined by the government, what are the parameters by which it plans to ensure compliance with the World Health Organization’s International Health Regulations and the Pandemic prevention, preparedness and response accord;
(h) how will the government implement a One Health approach as part of its public health planning in the future; and
(i) what is the extent to which efforts to reduce greenhouse gas emissions will be included in the government’s One Health approach?
And just before that COVID-related question, MP Lewis had also asked questions around MAID, recopied here for interest.
Q-29772 — September 17, 2024 — Ms. Lewis (Haldimand—Norfolk) — With regard to the administration of medical assistance in dying (MAID) in Canada:
(a) what is the current national standardized protocol for administering MAID;
(b) if the answer to (a) is that there is no standardized protocol, why not;
(c) how is the government evaluating the MAID protocols with regard to (i) their effectiveness, especially with regard to minimizing pain, (ii) procedure complications, (iii) procedure risks;
(d) since 2016, what independent medical research has the government either commissioned or collected that (i) evaluates MAID clinical practice and studies the evidence with regard to the medical risks and complications in MAID deaths carried out to date, particularly as they pertain to the medications used and dosages given, (ii) analyzes the totality of patients’ physical experiences and impacts;
(e) if the answer to (d) is none, what are the reasons;
(f) how many autopsy reports have been done on MAID patients;
(g) if the answer to (f) is none or unknown, what are the reasons;
(h) are any of the medications used to administer MAID in Canada used in executions in other countries;
(i) are any of the medications used to administer MAID illegal or prohibited in other countries;
(j) if the answers to (h) or (i) are in the affirmative, what are the details, including the (i) medication name, (ii) countries where it is used, (iii) method of use for execution or reasons the medication is illegal or prohibited;
(k) is the government aware of concerns from some medical professionals that the use of Midazolam and Propofol in MAID has the potential of causing a painful death even if it appears outwardly peaceful, and, if so, what is the government’s response;
(l) what is the government doing to investigate the concerns in (k);
(m) why does Statistics Canada not classify MAID as a cause of death; and
(n) when will the government resolve the death reporting incongruence between Statistics Canada and Health Canada?
MP Ted Falk re: Specifics on the Pfizer Contract
[Following the Covid Science asks: WHAT? did the mechanism of Order Paper Questions NOT exist three years ago? Did NONE of all of Canada’s MPs think to ask about this for three years? Or did someone ask but not receive any acceptable answers? I.e. was it considered a violation of the idea of proprietary information to actually answer these questions prior to this fall?]
Question No. 2745—September 16, 2024
Mr. Ted Falk:
With regard to the procurement, review and contents of the contract for the Pfizer COVID-19 mRNA vaccine signed by the former Minister of Public Services and Procurement in 2020:
(a) when did the former Minister of Public Services and Procurement, the former Minister of Health and Health Canada initially receive the Pfizer contract;
(b) which entities and agencies reviewed the contents of the Pfizer contract and who performed the review in each entity and agency;
(c) which entities and agencies approved the final terms of the Pfizer contract and who signed the approval in each entity and agency;
(d) did the contract specify whether their product was serialized by the manufacturer; (e) what is the purpose of product serialization by any drug manufacturer;
(f) if the answer to (d) is negative, why not;
(g) did the Pfizer contract provide unequivocal confirmation that their product was studied for its (i) efficacy to prevent infection of SARS-CoV-2, (ii) efficacy to prevent serious illness, (iii) efficacy to prevent hospitalization, (iv) efficacy to prevent death, (v) long-term side effects, (vi) ability to stop transmission of SARS-CoV-2, (vii) known adverse effects;
(h) did the contract state that the mRNA vaccine was tested for its ability to stop transmission of SARS-CoV-2 to others;
(i) with respect to the responses to (g) and (h), when was (i) Dr. Howard Njoo, (ii) Dr. Theresa Tam, (iii) Dr. Supriya Sharma, (iv) Dr. Caroline Quach-Thanh, (v) the Prime Minister, (vi) the Deputy Prime Minister and Minister of Finance, (vii) the former Minister of Health, (viii) the former Minister of Transport, provided this information;
(j) with respect to the responses to (g)(i) to (g)(vii), when was the Office of the Prime Minister informed about the limitations of the vaccine as listed in the Pfizer contract and who informed them;
and (k) who approved the communications plan after the contract was received and analyzed in early 2021 that would inform Canadians that the Pfizer product was "safe and effective" and prevented transmission of SARS-CoV-2 to others?
MP Colin Carrie re: Health Canada’s assessment of risks versus benefits for the COVID-19 vaccines
[Following the Covid Science asks AGAIN…. WHAT? did the mechanism of Order Paper Questions NOT exist three years ago? Did NONE of all of Canada’s MPs think to ask about this for three years? Or did someone ask but not receive any acceptable answers? I.e. was it considered a violation of the idea of proprietary information to actually answer these questions prior to this fall?]
Question No. 2809—September 16, 2024
Mr. Colin Carrie:
With regard to Health Canada’s (HC) assessment of risks versus benefits for the COVID-19 vaccines:
(a) did HC perform a formal analysis showing that the benefits of the COVID-19 vaccines outweigh the risks (i) at the time of interim order approval, (ii) at the time of authorization, under the amended Food and Drugs Regulation for September 2021, (iii) before the approval of each subsequent booster;
(b) if the answer to (a) is affirmative, who performed the analysis and what were the results of the analysis, specifying the benefits and risks (i) at the time of interim order approval, (ii) at the time of authorization, under the amended Food and Drugs Regulation for September 2021, (iii) before the approval of each subsequent booster;
(c) what specific scientific studies, real world data, and Canadian morbidity and mortality data were reviewed by HC to conclude the risks of the COVID-19 vaccines outweighed the risk of COVID-19 illness (i) at the time of interim order approval, (ii) at the time of authorization, under the amended Food and Drugs Regulation for September 2021, (iii) before the approval of each subsequent booster;
(d) what were the risks that HC determined for the COVID-19 vaccines compared to the risks of the COVID-19 illness (i) stratified across age groups, (ii) for the immunocompromised, (iii) for seniors with two or more comorbidities, (iv) for pregnant and lactating women, and what were these results;
(e) did HC use the Cleveland study entitled “Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine” by N. Shrestha et al to update their risk-benefit analysis of the current COVID-19 vaccine;
(f) if the answer to (e) is negative, why not;
(g) how were those individuals who received a COVID-19 vaccine classified as being “vaccinated” versus “unvaccinated” for the purposes of statistical analysis of clinical outcomes and vaccine efficacy by the following categories (i) less than two weeks after first dose of the primary series, (ii) between two weeks and three months after first dose of the primary series, (iii) less than two weeks after second dose of the primary series, (iv) more than two weeks after second dose of the primary series, (v) less than two weeks after any booster dose, (vi) more than six months after any booster dose;
(h) would the response in (g) be influenced by brand of COVID-19 vaccine, and, if so, how;
(i) for Canadian morbidity and mortality data presented to the Canadian public to illustrate the efficacy of the COVID-19 vaccines, how were the definitions from (g) and (h) used; and
(j) what data supported the definitions of the vaccination status as defined in (g)?
Response
(Return tabled)








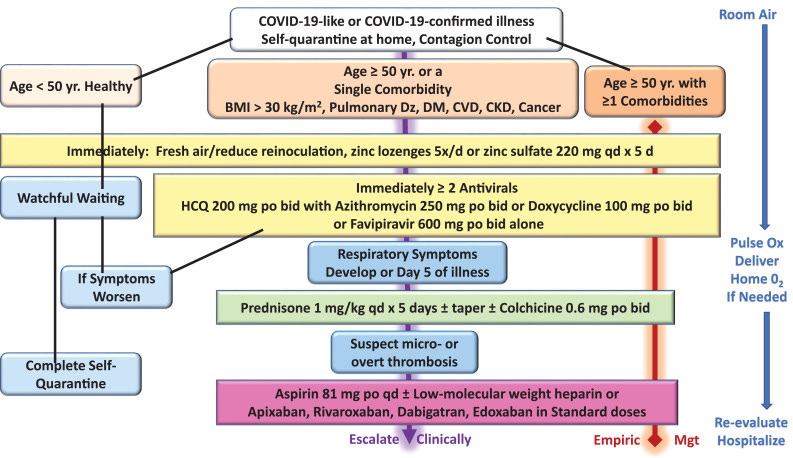





















This post is a valuable resource, but time-consuming to mine.
Wouldn’t we be better served by breaking this post into parts? Such as:
Part `. Intro & questions asked and answered.
Part 2. Questions asked and not answered
Part 3. Questions on euthanasia. Some experts have claimed that euthanasia recipients, suffer, excruciating and painful deaths while in a state of helpless paralysis. Others say it’s not possible. Answers here with respect to autopsies may unpack what is goes on in this regard.
(minor edits performed on Oct 11 2024)